Direct Methanol Fuel Cell (DMFC) owes its unique characteristics - high energy density, easy liquid fuel storage, low operation, and simplified system structure.
Working Principle of Direct Methanol Fuel Cell (DMFC):
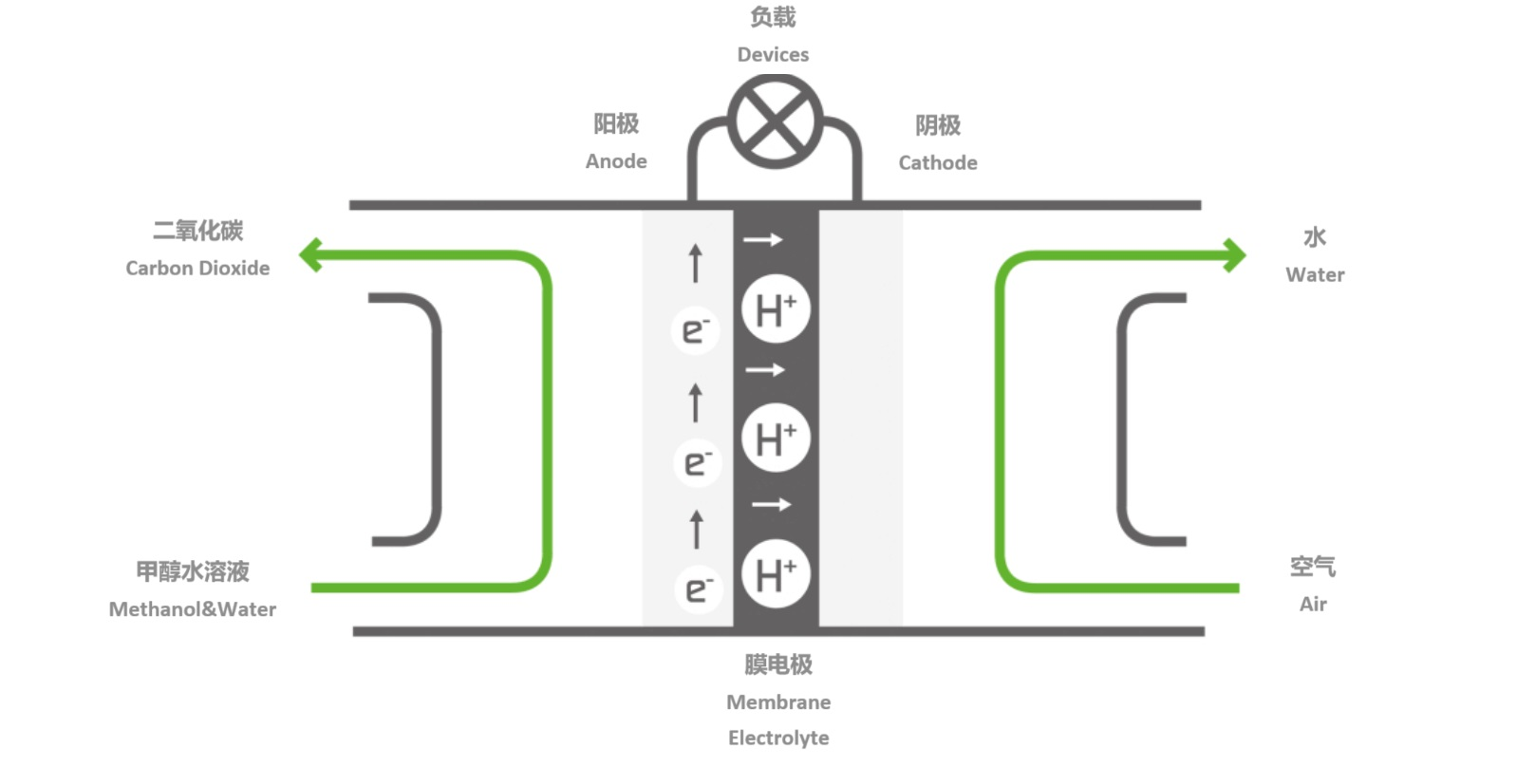
The design of DMFC is much like a battery. It has two electrodes separated by a membrane: anode and cathode. When combined with oxygen, the methanol fuel cell converts the fuel (methanol) into energy, hence the name. The only by-products of this chemical reaction are direct current, heat, water, and trace amounts of carbon dioxide.
How does the Direct Methanol Fuel Cell generate energy?
The electrochemical reaction of methanol and water occurs at the anode (methanol is oxidized), forming carbon dioxide, protons, and electrons.
Protons are generated at the anode and flow to the cathode through the polymer electrolyte, where they react with oxygen to form water.
The electrons generated at the anode carry the free energy change of the chemical reaction and pass through the external circuit, where they are used to generate electricity.
Like all fuel cells, direct methanol fuel cells have no moving parts. Therefore, they not only operate more quietly than internal combustion engines or gas turbines, but also have minimal mechanical friction and slight wear. Low maintenance requirements can reduce downtime, thereby reducing long-term operating costs.
For more information about Direct Methanol Fuel Cell technology:
https://en.wikipedia.org/wiki/Direct_methanol_fuel_cell